Archived
99/100
Average SEO score of top 100 sites: 75%
This webpage received an SEO score of 99 out of 100, which is higher than the average score of 75. Our analysis has identified 11 SEO issues that can be addressed to further enhance your website's performance and improve its search engine visibility.
11 Failed
0 Warnings
27 Passed
Common SEO issues
Score: 57
Failed: 4
Warnings: 0
Passed: 8
Google Search Results Preview Test
Desktop version
http://www.markbarbieri.info/Mark H. Barbieri, Quality Engineering, Regulatory Compliance, Quality Management SystemsQuality Engineering and Regulatory Compliance Professional. Experienced in medical device listing and registration, domestic and internationally. Extensive experience in quality management systems, ISO 9001, AS9100, ISO 13485, ISO 14971, EU MDD, FDA 21 CFR 820, Part 11, CMDR SOR-98/282, verification and validation, design controls, Mobile Medical Applications, Medical Device Data Systems, Medical Device Classifications, 510(k) submissions, and more.
Mobile versionhttp://www.markbarbieri.info/Mark H. Barbieri, Quality Engineering, Regulatory Compliance, Quality Management SystemsQuality Engineering and Regulatory Compliance Professional. Experienced in medical device listing and registration, domestic and internationally. Extensive experience in quality management systems, ISO 9001, AS9100, ISO 13485, ISO 14971, EU MDD, FDA 21 CFR 820, Part 11, CMDR SOR-98/282, verification and validation, design controls, Mobile Medical Applications, Medical Device Data Systems, Medical Device Classifications, 510(k) submissions, and more.
Keywords Cloud Test
Competitor Domains Test
Understand your competitors' SEO and backlink profile
Get related competitors and their domain authority score in relation to your domain.
Robots.txt Test
- This website is using a robots.txt file.
Sitemap Test
- Congratulations! We've found 1 sitemap file for your website:
Image Alt Test
- Your webpage has 6 'img' tags and none of them has the required 'alt' attribute.
Google Analytics Test
- Your website does not include Google Analytics tracker script or this script is not properly installed. You are advised to use Google Analytics (and properly install the tracker script) in order to get detailed statistics about your website's traffic and traffic sources.
Favicon Test
- We've found a favicon in your page's HTML code but it's not accessible.
Backlinks Test
Get a full and detailed list of your backlinks!
To view your total number of backlinks and referring domains, please sign-up for a free trial!
JS Error Test
- Congratulation! There is no occurrence of any severe JavaScript Errors in your web page.
Speed optimizations
Score: 32
Failed: 4
Warnings: 0
Passed: 1
HTML Page Size Test
- Your HTML size is 109.49 Kb and is over the average web page HTML size of 33 Kb.
This can lead to slower than average load times, lost visitors, and decreased revenue. Good steps to reduce HTML size include: using of HTML compression, CSS layouts, external style sheets, and moving javascript to external files.
HTML Compression/GZIP Test
- Congratulations! Your page is successfully compressed using gzip compression on your code.
Your HTML is compressed from 390.45 Kb to 109.49 Kb (72 % size savings). This helps ensure a faster loading web page and improved user experience.
Site Loading Speed Test
- Your site loading time is around 6.698 seconds and is over the average loading speed which is 5 seconds.
Accurate loading speed and website loading speed monitor
Get detailed and accurate loading speed reports for your websites and see how your pages are being loaded over time.
Register for free and use the Loading Speed Monitor from SEO Site Checkup Toolbox today and get valuable insights on how much time your customers need to wait until they see your page.
Page Objects Test
- Your page has more than 20 http requests, which can slow down page loading. You can try reducing http requests through various methods such as using text instead of images, using css sprites, using data URIs instead of images, or combining several external files together into one.
Image Caching Test
- Your site is not using expires headers for your images. An expires tag can help speed up the serving of your webpages for users that regularly visit your site and see the same images. Learn more about how to add expires headers to your images.
Server and security
Score: 0
Failed: 0
Warnings: 0
Passed: 2
URL Canonicalization Test
- http://markbarbieri.info and http://www.markbarbieri.info resolve to the same URL.
HTTPS Test
Plaintext Emails Test
- Congratulations! Your webpage does not include email addresses in plaintext.
Mobile usability
Score: 100
Failed: 0
Warnings: 0
Passed: 2
Media Query Responsive Test
- Congratulations, your website uses media query technique, which is the base for responsive design functionalities.
Mobile Snapshot Test
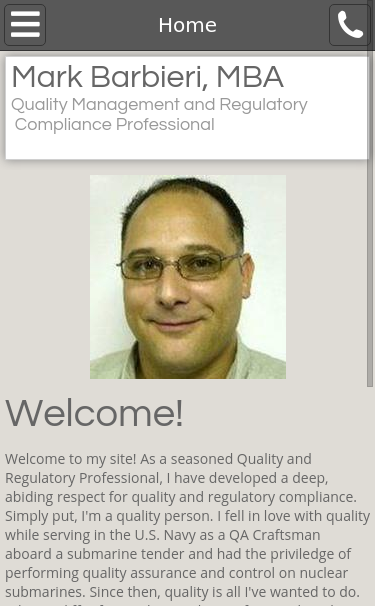
Advanced SEO
Score: 100
Failed: 0
Warnings: 0
Passed: 5
Structured Data Test
- Congratulations! Your website is using HTML Microdata specifications in order to markup structured data.
Product
- Pricing
- Free Tools
- Articles
- Login
- Free 7-Day Trial
© SEO Site Checkup 2020-2025 • All rights reserved